Rinsing Manual
Appendix A: Dragout Reduction Options
Topics covered in this appendix include:
The primary source of pollution in a metal finishing shop is the dragout of various processing baths into subsequent rinses. The amount of pollutants contributed by dragout depends on factors including the design of the racks or barrels carrying the parts to be plated, the shape of the parts, plating bath characteristics, and plating methods. Effective methods of reducing dragout formation are discussed in this section.
Plating solution control: As described below, several key solution factors affect the volume of dragout, including viscosity, surface tension, and temperature. By altering these parameters you can reduce dragout.
The viscosity of a plating process solution, a measure of its resistance to flow, determines how resistant the solution is to displacement by another liquid (in this case, rinse water). Viscosity results from the attractive forces between molecules, and will change with concentration or temperature. Higher viscosity results in more dragout. As a rule, increasing the concentration of a solution will increase its viscosity. This, in turn, increases dragout in two different ways -- a greater volume of liquid clings to the parts, and that volume contains a higher concentration of chemicals. It is often possible to reduce the plating bath concentration, which can provide very significant P2 benefits. For example, if two identical parts are immersed in separate chromium baths with concentrations of 53 ounces per gallon (397 g/L) and 33 ounces per gallon (247 g/L), respectively, the lower concentration bath will produce 73% less volume of dragout.
In addition to viscosity, the volume of solution that clings to a workpiece surface depends on surface tension. The attractive forces between molecules that result in viscosity are still present at the surface of a solution, but are unbalanced. At the surface, molecules are pulled toward the solution by other molecules just below the surface, but there is no corresponding attraction from the widely dispersed gas phase molecules on the other side. As a result, molecules at the surface are under tension and form a thin, skin-like layer that adjusts to create a minimum surface area. The force of surface tension has its most significant effect at the bottom edge of the part as it passes through and leaves the process solution, by decreasing the tendency of the solution to drip off the part. Surface tension can be reduced with the use of a wetting agent such as a surfactant. A typical plating bath solution has a surface tension close to that of pure water at room temperature or about 0.0050 lb/ft. The addition of very small amounts of surfactants can reduce surface tension considerably -- to as little as 0.0017 to 0.0024 lb/ft, effectively reducing dragout by about 50%.
Another factor that influences dragout volume is the temperature of the process solution. Higher temperatures create greater molecular agitation, which can partially overcome the attractive forces. As a result, increasing the temperature of a plating solution will cause a corresponding decrease in viscosity and surface tension, thereby reducing dragout volume.
Design and maintenance of racks and barrels:
Racks and barrels are designed to hold parts during processing and deliver sufficient electrical current to accomplish cleaning, coating or electroplating. There are obvious design factors involved, such as the materials of construction, which must be selected to conduct sufficient electricity and withstand the chemicals being used. An additional aspect of design that was at one time overlooked in the past is dragout.
Figure 2. Poor rack maintenance increases dragout because
solution is entrapped under broken rack coatings.For any given part, there is an alignment that will generate the least volume of dragout. The racks and hooks should allow workers to attach the parts in this orientation unless it presents problems with evenness of electrodeposit or other production quality issues. For example, parts with contoured surfaces should be held in a manner that solution is not entrapped, but rather flows off of the part and back into the tank. Flat shaped, rectangular parts should be held such that the shortest surface is vertical. In this position, dragout has less distance to flow before being released. Additional information on racking is presented below.
Customized racks or highly versatile racks should be used whenever possible and they should be designed to minimize dragout. Some experimentation can be performed to help make decisions regarding design.
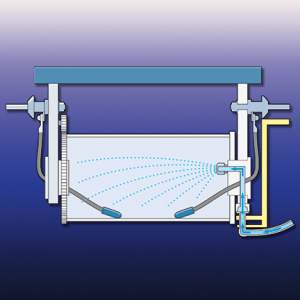
Figure 3. Innovative barrel designs include internal spray rinses
that increase rinse efficiency and reduce water use.Barrel design also has a significant impact on dragout. A 2002 study demonstrated that dragout rates for different barrel designs vary by 50%, with newer designs, aimed at dragout reduction, performing best. Barrel design aspects that affect dragout volume include barrel shape, hole shape and size, and material of construction (mesh-sided barrels performed better than solid-sided barrels). In recent years, methods for spray rinsing inside of barrels have been developed (see figure 3). Barrel operation also affects dragout. Even the best designed barrels should not be overloaded, which reduces draining, and they should be adequately rotated above the tank to allow the vast majority of dragout to be removed.
Rack and barrel maintenance are also important to minimizing dragout. During the EPA P2 project, testing showed that damaged plastisol rack coatings can trap a substantial amount of process solution. In one case, the concentration of chemicals dripping off a damaged rack was 10 times higher than for a well maintained rack.
Proper positioning of parts on racks:
The metal finisher's primary consideration in the positioning of workpieces on a rack is proper exposure of the parts to the anodes for optimal coverage and uniform thickness of the electrodeposit. But there is generally some room to maneuver while still producing acceptable parts. Within those limits, positioning should be adjusted to optimize drainage and rinsability. After all, not much is gained by producing well-plated parts whose surface is then damaged because of insufficient or inefficient rinsing. In addition, subsequent process tanks can be contaminated by drag-in of unremoved chemicals from the previous solution.

Figure 4. Avoid racking parts directly over one another
to prevent lengthening the drainage path of the plating solution.Several rules apply to the position of work on plating racks for dragout minimization. The basic principle, however, is that every object can be positioned in at least one way that will produce the minimum quantity of dragout. This position could be determined by experiment, but unless a significant number of similar items are to be plated, it may be advisable to follow these suggestions:
- Tilt all solid objects with plane or single-curved surfaces so that drainage is consolidated. Twist or turn the part so that drops of clinging fluid will merge and flow off the part by the quickest route.
- Avoid racking parts directly over one another to prevent lengthening the drainage path of the plating solution.
- Avoid table-like surfaces by tipping the part, but not at the expense of forming solution "pockets."
- Orient parts so that only a small surface area comes in contact with the liquid surface as it leaves the plating solution.
The velocity at which work is withdrawn from the process tank has a major effect on dragout volume. The faster an item is pulled out of the tank, the thicker the dragout layer will be, because the viscosity forces that would otherwise pull the liquid back into the bath as the part is raised do not have a chance to operate, and a much larger volume of liquid will remain clinging to the surface. The effect is so dramatic that one author suggests that most of the time allowed for withdrawing and draining the item should be used for withdrawal. An automatic machine that performs smooth, gradual withdrawal will usually drag out less solution per item racked than will manually operated equipment.
Figure 5. Reduce rack withdrawal speed to less than one foot per second. Draining/Rinsing Over the Plating Tank:
After a rack or barrel is removed from a process tank, the dragout drains from the item and it returns directly to the bath as long as the item is held over the tank. This simple method of direct dragout return can be maximized on a hand-line by installing a bar over the process line on which the operator can hang a rack or hook. On automatic machines, the unit can be programmed to increase dwell time above the process tank. For barrel operations, the barrel can be rotated over the process tank to help free the dragout.
Figure 6. Inadequate drain time drastically increases dragout losses.
In most cases, use a minimum drain time of 15 sec.The quality of a metal finishing process can be diminished if the dragout is permitted to dry on the part. This can cause staining, peeling, or passivation, or it may prevent complete rinsing. To increase the dragout removal rate over the process tank, the part can be rinsed with small amounts of water. The amount of water that can be used will depend on the water balance for a given process tank (use STERC's Evaporation Calculator to determine the rate of evaporation from plating tanks). The water balance is affected mostly by evaporation. Process solutions operated at temperatures greater than 120oF often have enough surface evaporation to permit rinsing directly over the tank without excessive dilution of the process bath. Note, however, that using this method may reduce or eliminate the potential benefit from other dragout recovery methods (e.g., use of a dragout tank).
Figure 7. Rotating barrels above process tanks improves drainage.
However, even with this practice, barrels take about 50% longer to drain than racks.Rinsing over the tank can be performed by flood rinsing (e.g., hose), spray rinsing, or fog rinsing. The use of flood rinsing is not practical except for very high temperature baths with high dragout rates. Spray rinsing uses less water than flood rinsing. With the proper selection of spray nozzles this can be a very efficient method of direct dragout return. Nozzle selection criteria should include flow rate, spray velocity, and spray pattern. Air-atomized sprays, which are generally more efficient than plain water sprays, should be considered. Sprays can be hand-held or mounted on the tank rim. For automatic plating machines, the sprays are usually controlled to operate only when the part exits the bath. Fog nozzles use less water (less than 0.1 gpm) and produce a finer spray than conventional nozzles.
In general, barrels take a longer time to drain than racks. Figure 7 shows a small barrel hoisted from and rotated above an alkaline cleaner tank. Rotating obviously improves the drainage rate, but the solution continues to drip even after 30 seconds. As discussed above, good barrel design can improve drainage rates and reduce the need for excessive drain times.
Figure 8. The TriRinse System provides an equivalent three-stage rinse
using only one tank and it combines the benefits of immersion and spray rinsing.
It works with either rack or barrel plating.One commercial barrel system provides the equivalent of a three-stage rinse system with a bath and two fresh water sprays using only a single tank (see video, figure 8). The system consists of a rinse tank, holding tank, pump, valves, and spray nozzles. The barrel is initially lowered into an immersion rinse and rotated to improve drag-out removal. Rinse water is then partially evacuated to the holding tank. The barrel, now out of solution, is rotated to improve drainage of rinse water. Fresh water (5 gal.) is then sprayed onto the rotating barrel. After the spray, the barrel is again rotated to improve drainage. Then, a second fresh water spray is applied and again the barrel is rotated. The barrel is removed from the tank and the level of water in the tank is raised by adding water from the holding tank and the system is ready for another cycle. With this example, only 10 gallons of fresh water is used per barrel and an equivalent volume is sent to wastewater treatment. If the rinse was operated as a single stage immersion rinse, more than 10 times as much wastewater would have been generated to attain the same level of cleaning.
Fog rinsing is used at exit stations of process tanks. A fine fog is sprayed on the work, diluting the dragout film and causing a run-back into the process solution. Fog rinsing is applied when process operating temperatures, high enough to produce a high evaporation rate, allow replacement water to be added to the process in this manner. Fog rinsing prevents dry-on patterns by cooling the workpieces, but it may preclude the use of a dragout tank as a recovery option. For fog rinsing to be effective, work must be withdrawn from the process tank at a slow rate.
The dragout tank is a rinse tank that initially is filled with pure water. As the plating line is operated, the dragout rinse tank remains stagnant and its chemical concentration increases as more work is processed. After a period of operation, if sufficient evaporation has taken place, a portion of the dragout tank solution can be added directly to the plating bath (e.g., using a transfer pump). Evaporation usually will be sufficient with baths such as chromium and nickel plating solutions that are operated at elevated temperatures. Low-temperature baths, such as cadmium or zinc plating solutions, have minimum surface evaporation and often their temperature cannot be significantly increased without degrading heat-sensitive additives. In such cases, an evaporation unit can be installed to concentrate the solution before returning it to the process tank.
STERC has an on-line tool, the Plating Tank Evaporation Calculator, for estimating the volume of evaporation. To use the tool, you input the process solution temperature, the surface dimensions of the tank and indicate whether or not that tank is aerated. The results are displayed in gallons per hour of evaporation.
Drag-in/Dragout Tanks: Drag-in/dragout rinsing (also referred to as double-dipping) is a variation of the dragout tank. It involves rinsing in the same solution before and after plating (see Exhibit 2-13). This can be achieved by using a single rinse tank or two hydraulically connected rinse tanks, usually located on opposite sides of the process tank. In the latter case, which is most applicable to automatic plating machines, the rinse water is recirculated between the two rinse tanks using a transfer pump to maintain equal concentration of chemicals in the tanks.
The advantage of a drag-in/dragout arrangement is that plating chemicals rather than pure rinse water are transferred into the process tank by incoming racks or barrels. This increases the recovery efficiency of the recovery rinse.
The drag-in/dragout system finds application with plating baths that have a low to moderate evaporation rate and especially with baths that tend to increase in volume (i.e., equivalent to a negative evaporation rate). This condition, referred to as "solution growth" is common to alkaline zinc/nickel baths, where the volume of drag-in (water from the preceding rinse) can be greater than the sum of dragout and evaporation.
A drain board or drip shield is a tilted surface placed between process and rinse tanks that catches the drips from racks or barrels as they are transferred between tanks, thus preventing the dragout from falling to the floor. The solution on the drain board returns to its original tank by gravity flow. The drain surface can be plastic or metal. For acid solutions, the best materials are vinyl chloride, polypropylene, polyethylene, and Teflon®-lined steel. Stainless steel should be used for hot alkaline solutions. It is important that the drain surface be positioned at an angle that allows the plating solution to return to the bath (i.e., rather than to the subsequent rinse).


Figure 9. Before and after installation of drain board.
Drain boards reduce dragout from entering rinse tanks and improve housekeeping.The effect of added drain boards can be very dramatic. Figure [X] shows before and after pictures of a zinc/nickel line where a drain board was added.
Direct dragout return: This section describes methods that recover dragout in tanks and then return it to the process tank. STERC has an on-line tool (Rinse Systems Calculator) that evaluates direct dragout return options and other multiple rinse tank configurations. To use the tool, some information concerning your process is needed, including process solution total dissolved solids (you can select some common processes from a pull-down tab), the regulated pollutant of interest, temperature, tank surface area, dragout rate, and rinse criterion. The output of the calculator is a graphical display showing the rinse flow rate needed to meet the rinse criterion for various tank configuration options.

